Von Hippel-Lindau Disease and Its Link to Advanced Renal Cell Carcinoma
 Oct, 19 2025
Oct, 19 2025
VHL-Associated RCC Treatment Selector
Treatment Selection Guide
This interactive tool helps clinicians select appropriate treatment options for patients with Von Hippel-Lindau disease-associated advanced renal cell carcinoma based on clinical characteristics and evidence-based recommendations.
Patient Assessment
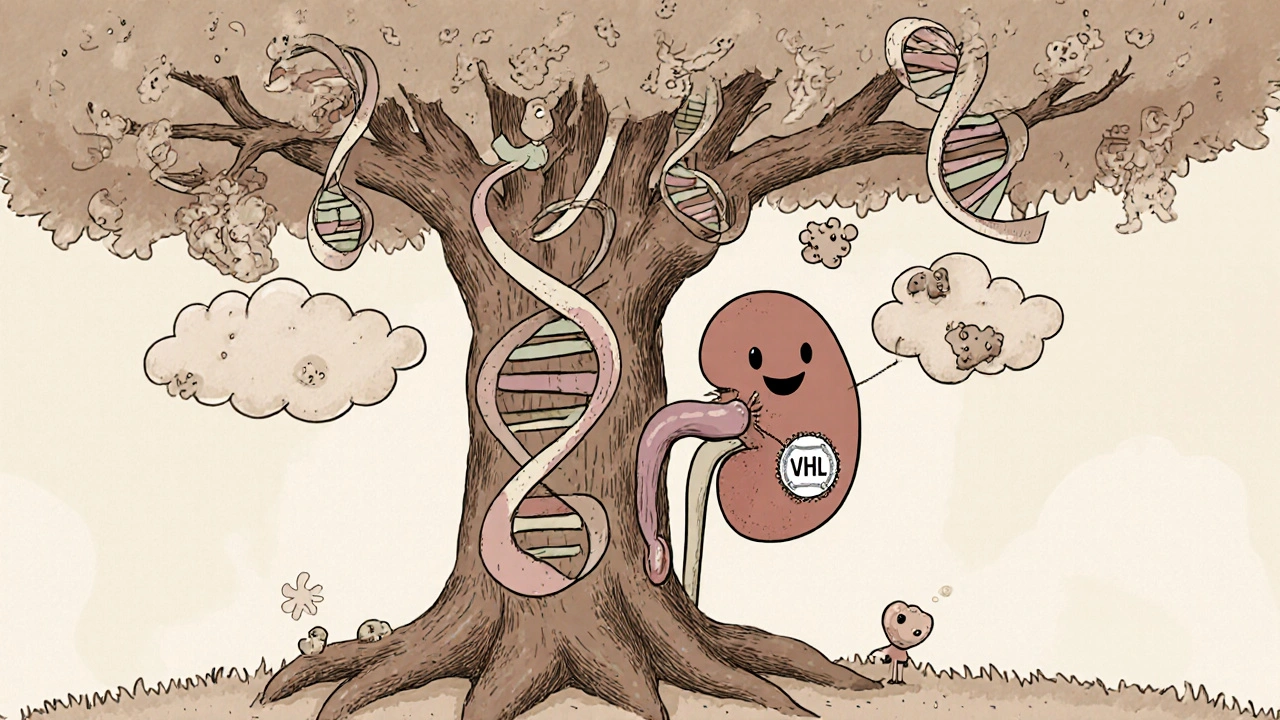
Von Hippel-Lindau disease is a rare inherited tumor syndrome caused by mutations in the VHL gene. People with VHL often develop cysts and tumors in multiple organs, and the kidneys are a frequent target. When cancer forms in the kidney, it is usually a renal cell carcinoma (RCC) that can progress to an advanced stage. Understanding how the VHL defect drives kidney cancer helps doctors choose the right surveillance and treatment strategies.
Von Hippel-Lindau disease may feel like a distant genetic term, but for patients it means a lifelong program of screening, surgery, and sometimes systemic therapy. Below we break down the biology, the clinical picture, and the latest therapeutic options for those facing advanced RCC linked to VHL.
Why the VHL Gene Matters in Kidney Cancer
The VHL protein normally tags a group of proteins called hypoxia‑inducible factors (HIF) for destruction when oxygen levels are normal. A broken VHL gene can’t do this, so HIF builds up even in well‑oxygenated tissue. Elevated HIF turns on the VEGF pathway, stimulating blood‑vessel growth and creating a fertile environment for tumor cells.
In the kidney, this cascade most often produces the clear‑cell subtype of RCC, the same histology seen in sporadic (non‑hereditary) cases. However, VHL‑associated tumors tend to appear at a younger age-often in the 30s or 40s-and may be multifocal or bilateral.
Clinical Presentation of VHL‑Associated Advanced RCC
- Multiple renal masses detected on surveillance imaging.
- Symptoms may include flank pain, hematuria, or a palpable mass, but many cases are found incidentally.
- Because patients are monitored from an early age, the disease is often caught before it spreads, yet some individuals still progress to stage III/IV.
When the cancer reaches an advanced stage, it can metastasize to lungs, bone, or liver, mirroring the pattern of sporadic RCC. The key difference lies in the underlying driver-persistent HIF signaling due to VHL loss.
Diagnosing Advanced RCC in VHL Patients
Imaging remains the cornerstone. Multiphasic contrast‑enhanced CT or MRI can map the size, number, and vascularity of renal lesions. For staging, a chest CT and bone scan (or PET‑CT) are added to look for distant spread.
Biopsy is usually reserved for atypical lesions, as the radiologic appearance of clear‑cell RCC is distinctive. Genetic testing confirms the germline VHL mutation, guiding both treatment and family counseling.
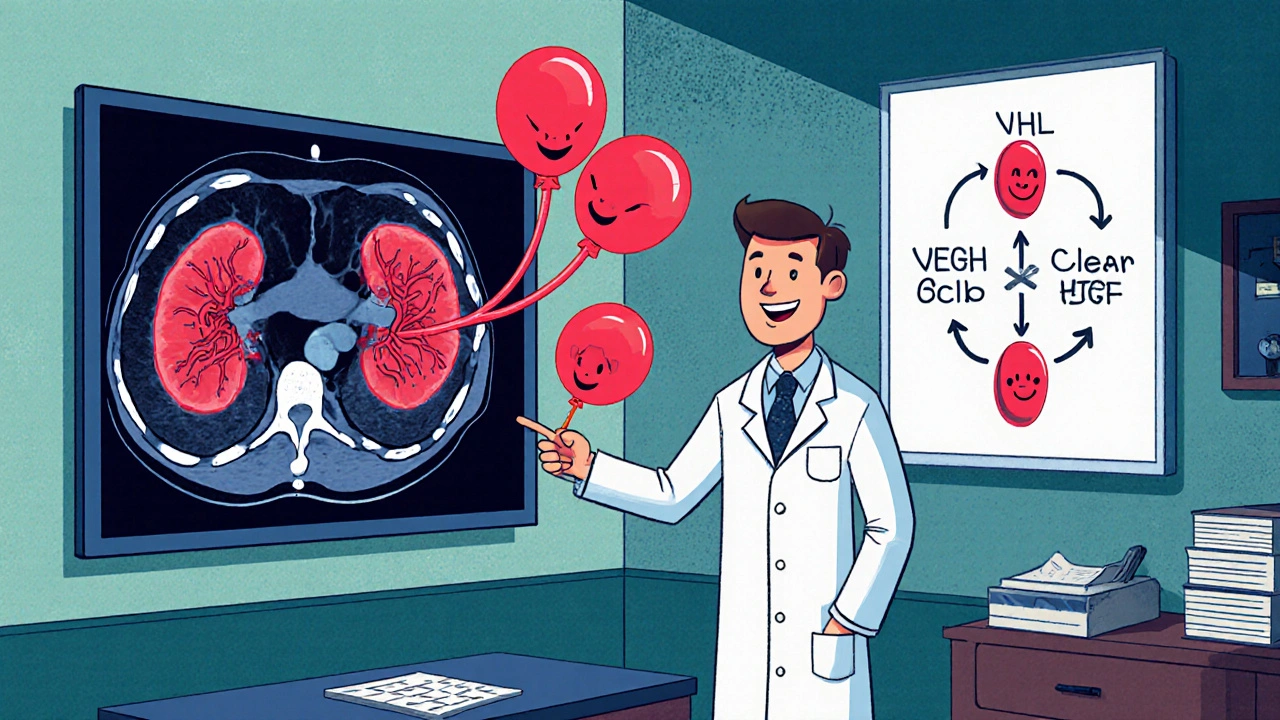
Treatment Landscape: How VHL Changes the Game
Therapy for advanced RCC has shifted from cytokines to targeted agents and immunotherapy. In VHL‑related disease, two pathways are especially relevant:
- Tyrosine kinase inhibitors (TKIs) that block VEGF receptors, such as sunitinib, pazopanib, and cabozantinib. These drugs directly counteract the VEGF surge caused by HIF.
- Immune checkpoint inhibitors (ICIs) that unleash T‑cells, most commonly pembrolizumab, nivolumab, or atezolizumab, often combined with a TKI for synergistic effect.
Clinical trials have shown that the TKI‑ICI combos improve progression‑free survival in both sporadic and VHL‑associated RCC, but patients with VHL mutations may experience a slightly better response to VEGF‑targeted therapy because their tumors rely heavily on that pathway.
Table: Key Differences Between Sporadic and VHL‑Associated Advanced RCC
| Feature | Sporadic Advanced RCC | VHL‑Associated Advanced RCC |
|---|---|---|
| Typical Age at Diagnosis | 55‑70 years | 30‑45 years |
| Genetic Driver | Various (VHL mutation 50% of clear‑cell cases) | Germline VHL loss |
| Common Histology | Clear‑cell (≈70%) | Clear‑cell (nearly 100%) |
| Typical Metastatic Sites | Lung, bone, liver, brain | Same pattern; sometimes earlier bone involvement |
| First‑Line Systemic Therapy | TKI + ICI combo (e.g., axitinib + pembrolizumab) | TKI + ICI combo (often cabozantinib + nivolumab) or TKI alone if contraindicated |
| Response Rate | ~40‑45% objective response | ~50‑55% objective response (higher to VEGF blockade) |
Surveillance and Genetic Counseling
Because VHL is inherited in an autosomal‑dominant pattern, every diagnosed patient should see a genetic counselor. First‑degree relatives have a 50% chance of carrying the mutation and benefit from early imaging.
Standard surveillance protocol (as of 2025) includes:
- Annual MRI of the brain and spine to catch hemangioblastomas.
- Abdominal MRI or CT every 1‑2 years to monitor renal lesions.
- Ophthalmic exam every 1‑2 years for retinal hemangiomas.
If a renal mass exceeds 3 cm or shows rapid growth, partial nephrectomy is preferred to preserve kidney function. For multifocal disease, staged or simultaneous surgeries may be planned, balancing oncologic control with quality of life.
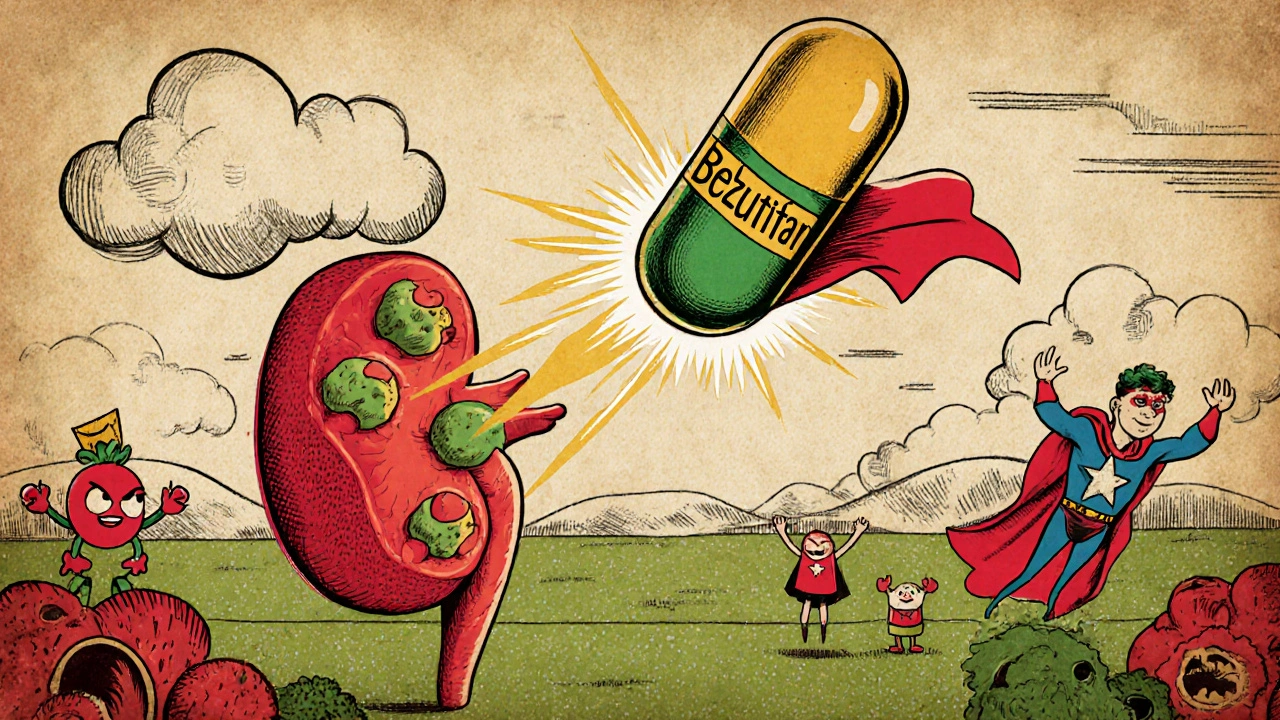
Emerging Therapies: HIF‑2α Inhibitors
Directly targeting the HIF pathway is an exciting frontier. The oral agent belzutifan received FDA approval in 2023 for VHL‑related renal lesions. It binds HIF‑2α, shutting down the downstream VEGF surge at its source. Early‑phase trials suggest a median progression‑free survival of 14 months in patients with advanced disease, outperforming historical TKI monotherapy.
Combination studies-belzutifan with pembrolizumab or with cabozantinib-are ongoing and could reshape first‑line standards.
Practical Checklist for Clinicians Managing VHL‑Associated Advanced RCC
- Confirm germline VHL mutation with genetic testing.
- Stage disease with contrast‑enhanced CT/MRI, chest imaging, and bone scan.
- Discuss systemic options: TKI + ICI combo or HIF‑2α inhibitor if eligible.
- Plan surgical intervention for lesions >3 cm when feasible.
- Enroll patient in a clinical trial if possible; many trials focus on HIF‑2α blockers.
- Arrange lifelong surveillance for other VHL manifestations and provide genetic counseling to family.
Frequently Asked Questions
What is the chance that a person with VHL will develop kidney cancer?
About 70‑80% of individuals with VHL develop renal cysts, and roughly 30‑40% of those cysts progress to clear‑cell renal cell carcinoma over their lifetime.
How does treatment differ between VHL‑related and sporadic advanced RCC?
Both groups receive VEGF‑targeted therapy and immunotherapy, but VHL‑associated tumors often respond better to pure VEGF inhibition because their growth is driven directly by HIF‑induced VEGF. HIF‑2α inhibitors are also an option unique to VHL patients.
Can family members be screened for VHL?
Yes. Since VHL is autosomal‑dominant, each first‑degree relative has a 50% risk. Genetic testing followed by baseline MRI of the abdomen and brain is recommended.
Is surgery still needed if systemic therapy is effective?
Surgery remains the best chance for cure of localized renal tumors. Systemic therapy can shrink tumors to make surgery feasible or can be used when surgery is not an option, but it rarely replaces the need for resection in resectable disease.
What are the most common side effects of HIF‑2α inhibitors like belzutifan?
Patients typically experience anemia, fatigue, and mild hypertension. The anemia is usually manageable with dose adjustments or erythropoietin‑stimulating agents.
Understanding the molecular bridge between VHL disease and advanced renal cell carcinoma equips patients and clinicians to act early, choose the right therapy, and monitor for other VHL‑related complications. With targeted drugs and emerging HIF‑2α inhibitors, outcomes are improving, turning what once felt like an inevitable decline into a manageable chronic condition.

Sunil Yathakula
October 19, 2025 AT 12:30Hey folks, just wanted to say that reading through this huge breakdown really hits close to home for anyone dealing with VHL. It can feel overwhelming, but remember you’re not alone – there’s a whole community out there supporting u. The way the article lays out the HIF‑VEGF cascade makes it easier to grasp why targeted therapies matter. Keep your head up and stay proactive with screenings; early detection is a game‑changer. Stay strong and keep fighting, the science is moving faster than ever!
Madhav Dasari
October 19, 2025 AT 15:20Wow, what a theatrical saga this VHL journey is! Imagine the drama of cells yelling “hypoxia” when oxygen is nowhere near scarce – it’s like a soap‑opera starring your kidneys. The cascade of HIF‑2α leading to VEGF is the plot twist nobody saw coming, and now we have the hero drugs swooping in. It’s inspiring to see patients getting a tailored script for their treatment, with TKIs and ICIs playing starring roles. Keep the optimism alive – every new trial is another act in this epic tale of triumph over genetics.
DHARMENDER BHATHAVAR
October 19, 2025 AT 18:06The mechanistic link between VHL loss and HIF‑2α activation underscores the therapeutic rationale for HIF‑2α inhibitors.
Kevin Sheehan
October 19, 2025 AT 22:16Let us not mince words: the philosophical underpinnings of targeting HIF‑2α are as aggressive as they are necessary. When the molecular landscape is skewed toward relentless angiogenesis, we must confront it head‑on with decisive pharmacologic force. The synergy of TKIs and ICIs is not a gentle compromise but a strategic onslaught. In the end, the pursuit of equilibrium in this chaotic system demands bold, unwavering action.
Penny Reeves
October 20, 2025 AT 02:26One must commend the authors for their exhaustive collation of data, yet the exposition suffers from occasional pedantry. The table, while informative, could benefit from more nuanced statistical commentary rather than rote comparisons. Moreover, the discussion of HIF‑2α inhibitors skirts the profundity of resistance mechanisms that merit deeper exploration. It is evident that the field is evolving, but a sharper analytical lens would elevate the discourse. Overall, a respectable synthesis, albeit tinged with superficiality.
Monika Bozkurt
October 20, 2025 AT 08:00Indeed, the pharmacodynamic properties of belzutifan warrant meticulous scrutiny, especially in the context of VHL‑associated oncogenesis. The inhibitor’s affinity for the HIF‑2α PAS‑B domain exemplifies rational drug design, integrating structural biology with clinical imperatives. While the efficacy signals are promising, one must remain vigilant regarding off‑target effects and longitudinal safety profiles. Nevertheless, the therapeutic paradigm shift toward molecularly targeted agents augurs well for patient outcomes. Continued interdisciplinary collaboration will be pivotal in refining treatment algorithms.
Christopher Burczyk
October 20, 2025 AT 14:56From an oncologic perspective, the integration of VEGF‑directed TKIs with checkpoint blockade represents the current gold standard for advanced RCC, irrespective of VHL status. The pharmacokinetic synergy observed in cabozantinib‑nivolumab regimens underscores the importance of dual pathway inhibition. Nonetheless, clinicians must balance efficacy with toxicity, tailoring regimens to individual comorbidity profiles. Emerging data on HIF‑2α antagonists may soon recalibrate first‑line strategies, particularly for germline VHL patients. Rigorous phase III trials will ultimately delineate the optimal sequencing of these modalities.
Nicole Boyle
October 20, 2025 AT 21:53Taking a step back, the epidemiological trends highlighted in this review reflect a broader shift toward precision medicine. The prevalence of multi‑organ manifestations in VHL underscores the necessity for integrated surveillance protocols. From a health‑systems viewpoint, allocating resources toward regular MRI screening can mitigate downstream oncologic burden. It’s fascinating to see how molecular diagnostics are reshaping clinical pathways. Overall, a solid overview with practical implications for multidisciplinary care.
Caroline Keller
October 21, 2025 AT 04:50Honestly the way some patients ignore regular scans is just reckless. It’s not just about you, it’s about families who suffer because of delayed diagnosis. We need to hold people accountable for their health choices.
dennis turcios
October 21, 2025 AT 11:46The analysis presented overlooks the heterogeneity of tumor microenvironments across VHL patients. A granular assessment of immune infiltration would have enriched the therapeutic outlook. Moreover, the cost‑effectiveness of belzutifan versus conventional TKIs remains inadequately addressed. While the data is compelling, a balanced critique must include real‑world adherence challenges. In sum, the manuscript offers a solid foundation but falls short of a comprehensive health‑economic evaluation.
Felix Chan
October 21, 2025 AT 20:06Great points! Let’s keep the conversation upbeat – staying positive really helps patients push through tough treatments. Keep sharing these insights, they’re super helpful.
Thokchom Imosana
October 22, 2025 AT 04:26There is a hidden narrative in the entire VHL discourse that most mainstream sources deliberately suppress. The fact that the biotech giants are pushing HIF‑2α inhibitors without disclosing the long‑term epigenetic repercussions is no coincidence. One must consider the broader agenda: the convergence of genomic editing, data mining, and pharmaceutical profit motives creates a feedback loop that benefits a select elite while ordinary patients become experimental subjects. The FDA’s rapid approval pathways, ostensibly designed for expedited access, often sidestep rigorous post‑marketing surveillance, allowing subtle adverse effects to fester unnoticed. Moreover, the emphasis on imaging surveillance can be viewed as a method to deepen patient dependence on costly radiologic services, inflating healthcare expenditure. It is not merely about therapeutic innovation; it is about control – controlling narratives, controlling data, controlling the very biology of populations. The association between VHL mutations and hypoxia mimetic pathways is leveraged to justify perpetual drug consumption, ensuring a steady revenue stream. While clinicians speak of “personalized medicine,” the reality may be a covertly orchestrated pharmacovigilance experiment where outcomes are monitored, but findings are selectively reported. Independent researchers who dare to question these paradigms often face funding cuts or academic ostracism, further consolidating the power structure. The synergy between big pharma and regulatory bodies creates an ecosystem where transparency is sacrificed at the altar of progress. Consequently, patients and families are entangled in a web of clinical trials that are marketed as cutting‑edge solutions but may serve as data collection platforms for undisclosed purposes. The timing of belzutifan’s approval, coinciding with broader legislative pushes for genomic data sharing, raises eyebrows. As the scientific community embraces CRISPR and other gene‑editing technologies, the groundwork laid by the VHL research could be repurposed for more invasive interventions. In essence, what is presented as a breakthrough in oncology might also be a testing ground for future biotechnological control mechanisms. Vigilance, critical appraisal, and demand for full disclosure are essential if we are to safeguard patient autonomy and prevent the covert commodification of our genetic selves.