How Clinician Communication Shapes Patient Trust in Generic Medications
 Jan, 3 2026
Jan, 3 2026
When your doctor hands you a prescription for a generic drug, what do you think? Maybe you wonder if it’s really the same. Maybe you’ve heard stories about generics causing side effects or not working as well. You’re not alone. But here’s the truth: clinician communication is the single biggest factor deciding whether you’ll take that generic pill-or refuse it altogether.
Why Patients Doubt Generics (It’s Not About the Science)
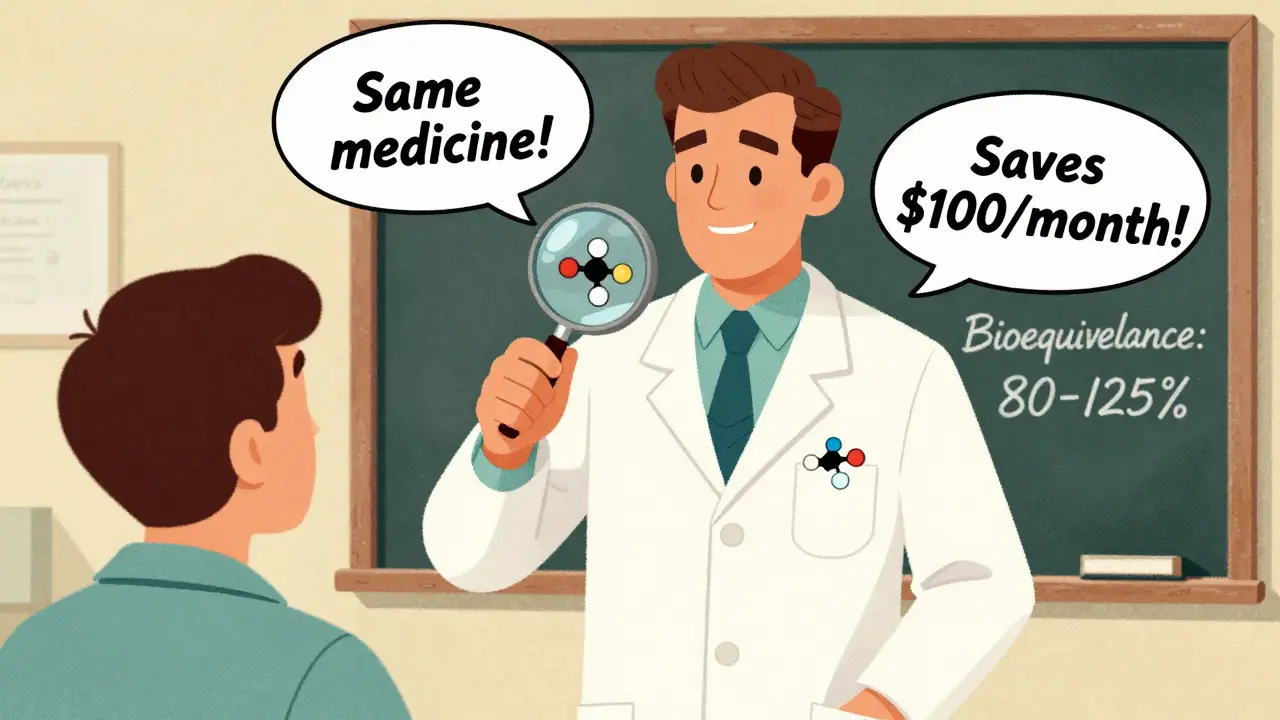
The science is clear. The FDA requires generic drugs to be bioequivalent to brand-name versions. That means they deliver the same active ingredient at the same rate and strength, within an 80-125% range. That’s not a guess. It’s a strict, tested standard. Yet, nearly 30% of patients still believe brand-name drugs work better. Why? It’s not because the pills are different. It’s because they weren’t told otherwise. A 2011 study of nearly 2,000 patients found that whether someone accepted a generic had almost nothing to do with cost, safety concerns, or even personal beliefs about generics. The only thing that mattered? Whether their doctor or pharmacist actually talked to them about it. Patients who got a clear explanation were 37% more likely to stick with the generic. Those who didn’t? They often stopped taking it-or blamed the drug when things went wrong.The Nocebo Effect: When Expectations Make You Sick
Your brain is powerful. If you believe a pill will make you feel bad, it often will-even if the pill is identical to one you’ve taken before. This is called the nocebo effect. It’s the dark twin of the placebo effect. A 2019 JAMA study tracked 412 patients with chronic conditions after switching to generics. Those who got a simple, standard message like “We’re switching you to a cheaper version” reported more headaches, dizziness, and fatigue. But patients who got a detailed explanation-told that the FDA requires generics to match brand drugs exactly, that the active ingredient is identical, and that side effects are rare-reported 28% fewer symptoms. Not because the drug changed. Because their expectations did. One patient on Reddit shared: “My cardiologist sat down, showed me the FDA bioequivalence chart, and said, ‘I take generics too.’ I’ve been on it for two years. No issues.” Contrast that with a Healthgrades review: “My pharmacist just handed me a new pill. When I got headaches, he said, ‘Some people react to generics.’ I stopped taking it for three weeks.”What Effective Communication Actually Sounds Like
It’s not enough to say, “This is cheaper.” That’s a sales pitch, not a clinical conversation. Effective communication has four key parts:- Explain bioequivalence: “The FDA requires this generic to work exactly like the brand. It’s held to the same strict standard-80% to 125% of the same effect.”
- Confirm identical active ingredients: “The medicine inside is the same. The color or shape doesn’t change how it works.”
- Highlight savings: “This saves you about 80%-that’s $100 a month off your prescription.”
- Address fears head-on: “Some people worry about generics because they’ve heard stories. But the science says they’re the same. If you feel different, let’s talk. It might be something else.”
Who’s Most at Risk-and Why
Not everyone reacts the same. A 2016 NIH survey found that non-Caucasian patients were 1.7 times more likely to distrust generics. Patients earning under $30,000 a year were 2.3 times more likely to insist on brand-name drugs. That’s not about intelligence. It’s about history. In communities with less access to reliable healthcare, people have been sold on the idea that “better” means “more expensive.” Marketing campaigns from brand-name companies have reinforced this for decades. One 2021 study showed that culturally tailored communication-using language, examples, and trusted messengers from the patient’s own background-reduced skepticism by 41%. A pharmacist in Milwaukee told me about a patient who refused a generic for high blood pressure because “the last time I took a cheap pill, I almost passed out.” Turns out, that was years ago, and the brand had changed. But the memory stuck. The pharmacist didn’t argue. She asked, “What happened?” Then she showed him the FDA’s generic approval process in his language. He took the pill. His blood pressure stabilized.When Both Doctor and Pharmacist Speak Up
Communication works best when it’s consistent. A 2021 study found that patients who heard the same message from both their doctor and pharmacist had a 92% acceptance rate. If only one spoke up? It dropped to 76%. If neither did? Only 61% accepted the generic. That’s why Kaiser Permanente’s “Generic First” program works. Every provider gets trained. Every script is standardized. Every EHR prompts the clinician to confirm they’ve explained the switch. Result? 94% of prescriptions filled are generics. And they’ve saved $1.2 billion a year.Barriers Are Real-But Fixable
Doctors are busy. A 2020 study found the average time spent explaining generics? Just 1.2 minutes. Many clinicians don’t know the exact bioequivalence range. Only 54% got it right in a 2019 survey. And 39% admit they’re unsure if generics work for conditions like epilepsy or thyroid disease. The fix? Training. The American Pharmacists Association created a 15-minute toolkit. After using it, pharmacists cut their explanation time by 38%-and patient understanding jumped from 42% to 87%. That’s not magic. That’s structure.
The Bigger Picture: Generics Are Saving Billions
In 2022, 90% of all prescriptions filled in the U.S. were generics. But they made up only 23% of total drug spending. That’s $37 billion saved in one year. Yet, brand-name preference requests have climbed from 12% in 2010 to 23% in 2022. Why? Because communication hasn’t kept up. The FDA, AMA, and CMS are now pushing for better practices. Epic’s new “Generic Confidence Score” in electronic health records now prompts doctors to check off four key points before prescribing. Medicare Part D is testing reimbursement tied to communication quality. The CDC plans to make this part of national health literacy standards by 2025.What You Can Do
If you’re a patient: Ask. “Is this generic the same as the brand? How do you know?” Don’t be shy. You’re not questioning your doctor-you’re asking for clarity. If you’re a clinician: Don’t assume they know. Don’t say “It’s cheaper.” Say “It’s the same medicine, approved by the FDA, and saves you $100 a month.” Write it down. Repeat it. Make it part of your routine. The science isn’t the problem. The silence is.Are generic medications really as effective as brand-name drugs?
Yes. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also meet strict bioequivalence standards-delivering the same amount of medicine into your bloodstream within an 80-125% range. Thousands of studies confirm they work the same way. The only differences are inactive ingredients like color or filler, which don’t affect how the drug works.
Why do some people feel worse after switching to a generic?
It’s often the nocebo effect-when expecting side effects leads to experiencing them. If you’ve been told generics are inferior, or if your provider didn’t explain the switch clearly, your brain may interpret normal fluctuations as side effects. Studies show patients who receive clear, confident explanations report fewer symptoms after switching, even though the drug is identical.
Can pharmacists explain generics better than doctors?
Both play important roles, but pharmacists often have more time to explain. A 2020 survey found that 53.7% of patients said their doctor never discussed generics, while 52% said the same about their pharmacist. But when pharmacists do explain-especially using FDA data and clear language-acceptance rates jump from 68% to 92%. The best outcomes happen when both provider and pharmacist are aligned.
Is it true that some generics aren’t as good?
The FDA approves all generics before they reach the market. There have been rare cases of manufacturing issues-like the 2012 bupropion controversy-but these are exceptions, not the rule. When problems occur, the FDA pulls the product. Most generics on the market today are safe and effective. The bigger issue isn’t quality-it’s perception, fueled by poor communication.
How can I tell if my doctor is giving me a good explanation?
A good explanation includes: 1) confirmation that the generic has the same active ingredient, 2) mention of FDA bioequivalence standards, 3) clear statement that it’s just as effective, and 4) an invitation to ask questions. If they just say, “This is cheaper,” or avoid the topic, ask for more. You have a right to understand your treatment.

Kerry Howarth
January 3, 2026 AT 18:59Doctors need to stop treating patients like they’re dumb. If you say ‘it’s cheaper,’ of course they’re gonna doubt it. Just say it’s the same medicine, FDA-approved, and you take it too. Done.
Tiffany Channell
January 4, 2026 AT 17:10Let’s be real-this whole ‘nocebo effect’ narrative is just a way for pharma and insurers to gaslight patients into accepting subpar drugs. If someone feels worse, maybe it’s not in their head. Maybe the fillers are different, and the body reacts. The FDA’s 80-125% range isn’t ‘exact.’ It’s a loophole.
Joy F
January 5, 2026 AT 18:43Here’s the ontological truth: we don’t just take pills-we take narratives. The pill is a symbol. The brand name is mythos. The generic? It’s the ghost of capitalism whispering, ‘You deserve less.’ And when your doctor says ‘it’s the same,’ you hear ‘you’re being optimized.’ That’s why the nocebo works-it’s not biology, it’s betrayal. The system trained us to equate cost with quality, and now our bodies are the collateral.
So yes, communication fixes it. But only if the clinician is willing to admit the system is rigged. Otherwise, it’s just performative empathy wrapped in FDA jargon.
Haley Parizo
January 6, 2026 AT 15:54Stop acting like this is about ‘communication.’ This is about power. The pharmaceutical industry spends billions marketing brand names as superior. They pay doctors to push them. They lobby against generic labeling laws. And now we’re supposed to be impressed that a doctor said ‘it’s the same’? That’s not communication-that’s damage control. The real fix is breaking the monopoly, not training doctors to be better salespeople for corporate greed.
And don’t even get me started on how non-white patients are 1.7x more likely to distrust generics. That’s not ‘history’-that’s systemic racism in pill form. You think a 15-minute toolkit fixes centuries of medical exploitation? Please.
Ian Detrick
January 8, 2026 AT 11:39I’ve been on generics for years-blood pressure, cholesterol, even my thyroid med. Never had an issue. But I’ll tell you what changed everything: my doctor didn’t just say ‘it’s cheaper.’ He pulled up the FDA chart on his tablet, showed me the bioequivalence range, and said, ‘I give this to my mom.’ That’s it. No fluff. Just truth and a little humanity. I trusted him because he didn’t treat me like a number.
People don’t need lectures. They need to feel like their concerns are real. And when you validate that-then give them facts-it sticks.
Angela Fisher
January 9, 2026 AT 05:41Okay but have you heard about the 2018 incident where a generic version of a seizure med caused 200+ hospitalizations? They pulled it, but the FDA didn’t tell anyone. And now they want us to believe generics are ‘just as good’? That’s not trust-that’s brainwashing. And don’t even get me started on the fillers-talc, gluten, dyes-they’re putting stuff in those pills that’s not even tested for long-term effects. My cousin’s anxiety got worse after switching. They said ‘it’s in your head.’ Turns out she was allergic to the dye. They don’t even list fillers on the label. This is a cover-up.
They’re pushing generics because they’re trying to kill the middle class. You think your $100 savings matters when your body is breaking down? They want you dependent on cheap meds so you don’t go to the doctor. And when you do, they blame you for ‘not taking it right.’ I’m not taking any more of their poison.
My sister died because they switched her to a generic heart med. The death certificate said ‘cardiac arrest.’ But the pharmacy log showed it was the 3rd batch they’d given her that month. They don’t track that. They don’t care. And now you want me to believe a doctor’s 1.2-minute speech fixes this?
They’re lying. All of them. Doctors. Pharmacists. FDA. You think this is about communication? No. It’s about profit. And you’re being used.
Stay off generics. Always ask for the brand. Even if it costs more. Your life is worth more than a $100 bill.
Neela Sharma
January 9, 2026 AT 16:45In India we call generics ‘sasta dawa’-cheap medicine. But our grandmothers trust them more than brand names. Why? Because they’ve seen real poverty. They know the difference between ‘cheap’ and ‘false.’ A good doctor doesn’t explain science-they explain survival. When my aunt’s BP pill switched, our village pharmacist sat with her for an hour, showed her the same tablet from different boxes, and said, ‘The soul inside is the same. Only the wrapper changed.’ She took it. Her BP stayed stable. No jargon. Just truth wrapped in love.
Liam Tanner
January 10, 2026 AT 04:52One thing this post misses: the role of community. In my clinic, we started having monthly ‘Medication Q&A’ sessions-open floor, no pressure. Patients bring their pill bottles. We compare brand vs generic side by side. We let them hold them, smell them, ask anything. One woman cried because she thought she’d been poisoned for a year. Turns out, she just didn’t know the color change was normal. Connection > brochures.
Palesa Makuru
January 10, 2026 AT 14:13Look, I get it. You want to feel like a good clinician. You want to pat yourself on the back for ‘communicating better.’ But here’s the truth-you’re not fixing the system. You’re just making it prettier. You think a 15-minute toolkit solves decades of medical neglect? You think a patient who’s been gaslit by insurance companies and pharma ads is going to trust you because you said ‘it’s the same’? Please. Real change means paying doctors to spend time, banning direct-to-consumer ads, and forcing transparency on fillers. Not a checklist. Not a script. Real justice.